The vibrant colors produced when certain chemicals are heated have always fascinated scientists and casual observers alike. But why do chemicals have to be heated to emit color? The answer lies within the intricate world of atomic structure and energy transitions. Let’s delve into the fascinating science behind this phenomenon.
The Science Behind Heating and Color Emission
At the heart of this colorful display is the relationship between heat, energy, and the behavior of electrons within atoms. Each atom has electrons orbiting its nucleus at specific energy levels. When heat is applied to a chemical, the atoms absorb this energy. This absorbed energy excites the electrons, causing them to jump to higher energy levels. Think of it like giving a boost to an electron, allowing it to momentarily occupy a more energetic position.
However, this energized state is unstable. The electrons prefer to reside in their original, lower energy levels. As the excited electrons fall back to their ground state, they release the absorbed energy as photons of light. The energy of these emitted photons determines the color we perceive. Higher energy photons correspond to colors like blue and violet, while lower energy photons correspond to colors like red and orange.
After this paragraph, insert this shortcode: 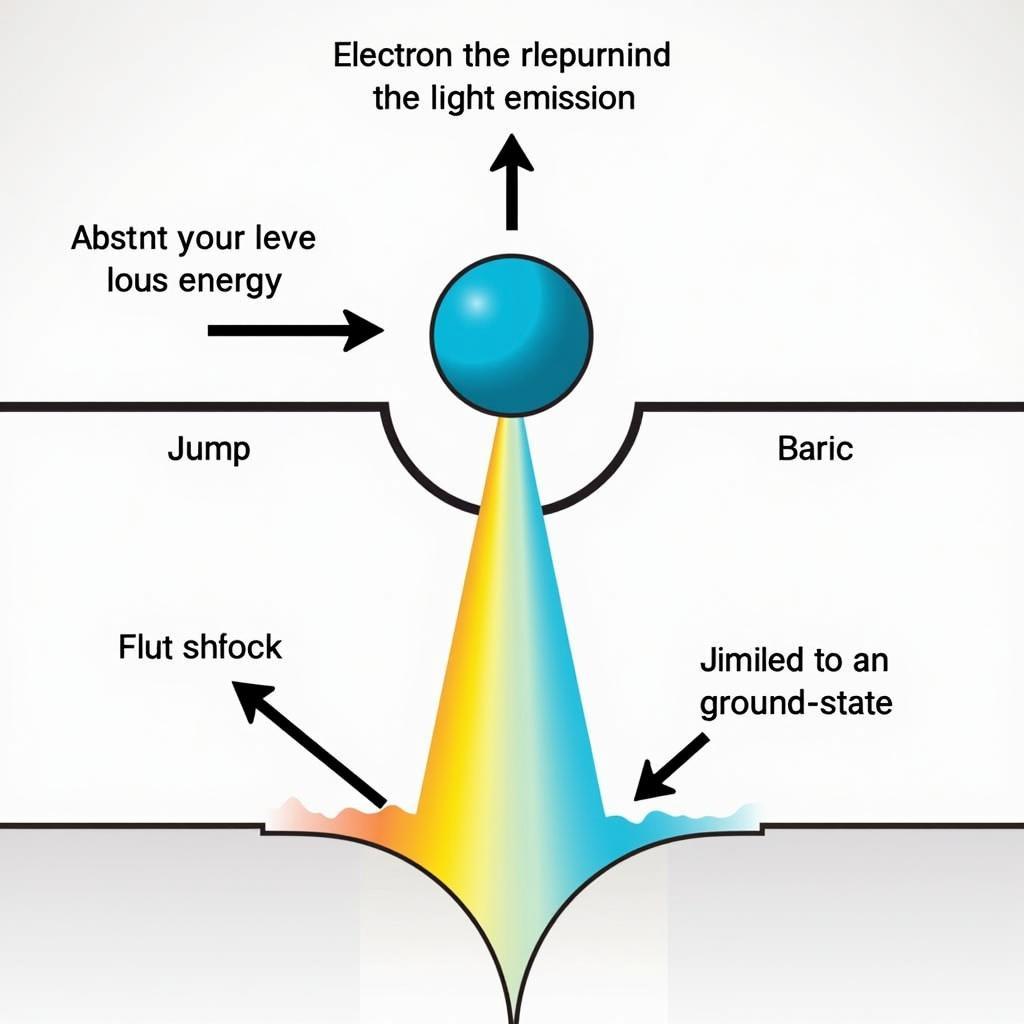{width=1024 height=1024}
Different chemicals have unique electron configurations and energy level spacing. This means each chemical will emit a specific color when heated, acting like a unique fingerprint. This phenomenon is utilized in various applications, from fireworks displays to analytical chemistry. For example, the distinct colors observed in fireworks are a result of carefully chosen metal salts emitting specific colors when heated.
How Different Chemicals Produce Different Colors
The specific color emitted by a heated chemical is dictated by the energy difference between the excited state and the ground state of its electrons. This difference varies greatly between elements and compounds, leading to a wide spectrum of observable colors. For instance, copper compounds often produce a green flame, while strontium compounds are known for their vibrant red hues. You can explore creating different fire colors at home, though it’s crucial to do so safely. For more details on achieving vibrant flames, check out how to make different colors of fire.
Factors Influencing Color Emission
Beyond the chemical composition itself, several other factors can influence the observed color. Temperature plays a significant role, with higher temperatures generally leading to more intense and sometimes even different colors. The presence of other substances can also affect the color, as they may interact with the emitting chemical or alter the flame’s temperature.
After this paragraph, insert this shortcode: “
Practical Applications of Color Emission from Heated Chemicals
The principle of color emission upon heating has numerous practical applications across various fields. In analytical chemistry, flame tests are used to identify the presence of certain metals in a sample. In pyrotechnics, the vibrant colors of fireworks are achieved by carefully combining different metal salts to produce desired effects. You can even discover how to change the color of fire with household items.
The Role of Spectroscopy
Spectroscopy, a technique that analyzes the light emitted or absorbed by substances, relies heavily on this phenomenon. By studying the specific wavelengths of light emitted by a heated sample, scientists can identify the elements present and determine their concentrations. This technique is crucial in fields ranging from astronomy to materials science.
Conclusion
The emission of color by heated chemicals is a fascinating phenomenon rooted in the fundamental principles of atomic structure and energy. The unique electron configurations of different elements and compounds dictate the specific colors they emit when heated, leading to a wide range of applications from analytical chemistry to the dazzling displays of fireworks. Understanding this fundamental principle opens up a world of color and provides insights into the intricate workings of the universe at the atomic level. Why do chemicals have to be heated to emit color? The answer lies in the dance of electrons and the energy they release as light.
FAQ
- What causes the color we see when chemicals are heated?
- Do all chemicals emit color when heated?
- Why do different chemicals emit different colors?
- How is this principle used in fireworks?
- What is spectroscopy, and how does it relate to color emission?
- Can the color of the emitted light change with temperature?
- Are there any safety precautions to consider when heating chemicals?
Need Help with Your Color Projects?
Contact us for expert advice on color selection, paint application, and design.
Phone: 0373298888
Email: [email protected]
Address: 86 Cầu Giấy, Hà Nội.
Our customer service team is available 24/7.


