Fireworks paint the night sky with vibrant hues, transforming celebrations into breathtaking spectacles. But how do fireworks get their color? The secret lies in a fascinating blend of chemistry and artistry, carefully orchestrated to produce the dazzling displays we all enjoy.
Similar to what determines the color of an object, the vibrant colors of fireworks are a result of meticulously selected chemical compounds, specifically metal salts. These salts, when heated to high temperatures during the firework explosion, emit light at specific wavelengths, creating the brilliant colors we see. Different metal salts produce different colors, allowing firework designers to create a wide spectrum of hues.
The Science Behind the Sparkle: Metal Salts and Light Emission
The core of a firework typically contains black powder for lift charge and a separate compartment packed with small pellets called “stars.” These stars are the key to the firework’s color. Each star is a mixture of a metal-containing salt, a fuel, a binder, and an oxidizing agent. The fuel and oxidizing agent react to create the heat and energy needed for the explosion, while the binder holds everything together.
When the firework explodes, the stars are ignited, and the metal salts within them are heated to incredibly high temperatures. This intense heat excites the electrons within the metal atoms, causing them to jump to higher energy levels. As these electrons return to their original, lower energy levels, they release energy in the form of light. The specific wavelength of light emitted, and therefore the color we see, is determined by the type of metal present in the salt.
Decoding the Firework Rainbow: Which Metal Creates Which Color?
The artistry of firework design lies in the careful selection and combination of metal salts to achieve the desired color palette. Here are some common examples:
- Red: Strontium salts, such as strontium carbonate, are responsible for the vibrant reds in fireworks.
- Orange: A combination of calcium salts, like calcium chloride, contributes to the warm orange hues.
- Yellow: Sodium salts, particularly sodium nitrate, produce the bright, intense yellows we see in many firework displays.
- Green: Barium salts, like barium chloride, are the key to creating the vivid greens that light up the night sky.
- Blue: Copper salts, such as copper(I) chloride, are used to create the challenging yet beautiful blue hues in fireworks.
- Violet: A mixture of strontium salts for red and copper salts for blue creates the vibrant violet shades.
Beyond the Basics: Achieving Complex Colors and Effects
Firework designers don’t stop at single colors. They often combine different metal salts within a single star to create more complex colors and effects. For instance, mixing strontium and copper salts can produce shades of purple or pink. The precise proportions of each salt are crucial in achieving the desired final color.
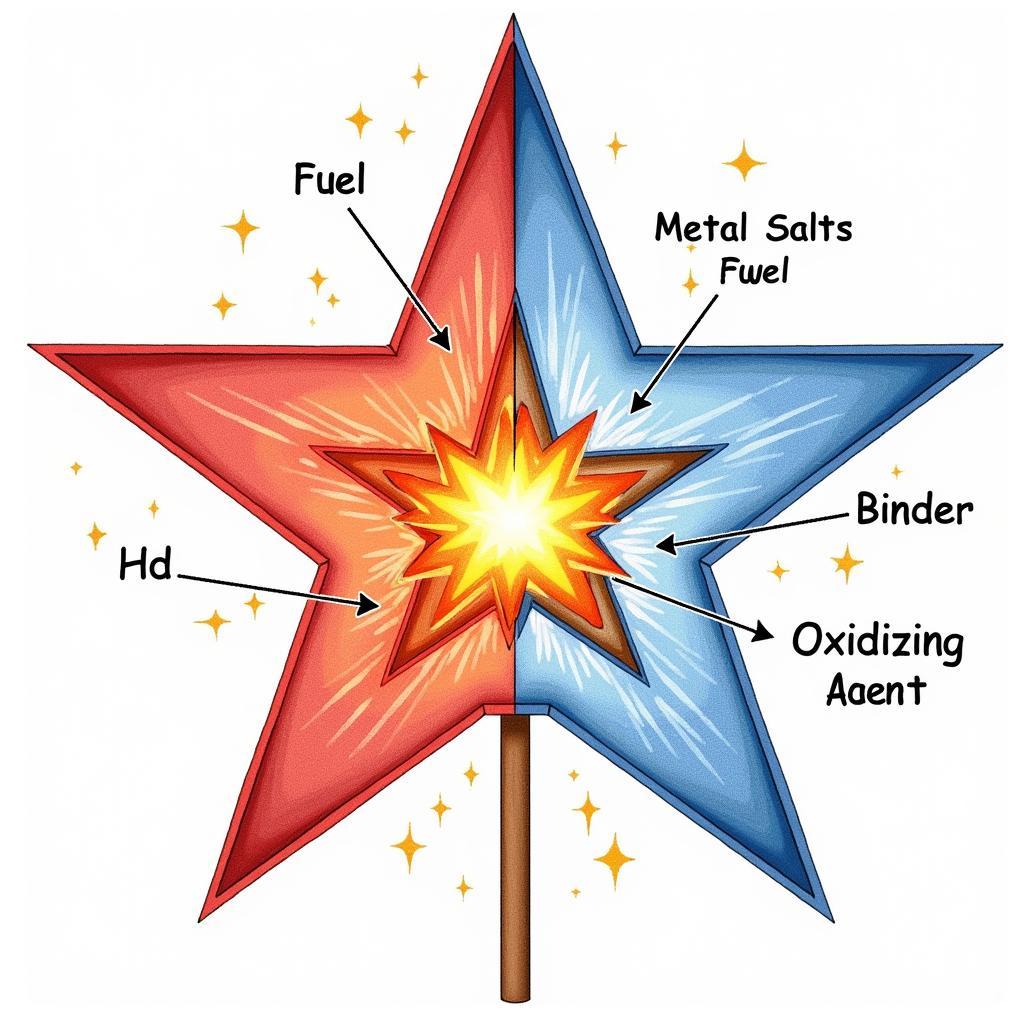 Firework Star Composition and Chemical Reaction
Firework Star Composition and Chemical Reaction
Furthermore, the size and shape of the stars, as well as the way they are packed within the firework shell, can influence the final effect. Larger stars burn longer and produce larger bursts of color, while smaller stars create a more sparkling, twinkling effect.
Are there ticks in colorado? This question might seem unrelated, but it highlights the importance of understanding the specific environment and regulations related to fireworks usage, similar to the regulations regarding various activities in different locations. Always check local laws and guidelines before using fireworks.
What Makes Fireworks So Bright?
The intensity of the light emitted by fireworks isn’t solely dependent on the metal salts. The addition of other chemicals, such as chlorine donors, can enhance the brightness and intensity of the colors. These chlorine donors help to create volatile metal chlorides, which are more efficient at emitting light than the original metal salts.
How To Make a Fire Different Colors: Exploring Similar Principles
The principle of using metal salts to create color isn’t limited to fireworks. You can observe similar effects in other contexts, such as how to make a fire different colors. Introducing certain metal salts to a flame can alter its color, demonstrating the same scientific principle at play. Can you smoke in public in colorado? Similar to firework regulations, understanding local laws regarding public smoking is essential.
The Art of the Display: Combining Color, Shape, and Timing
The magic of fireworks lies not just in the individual colors, but in how those colors are combined with different shapes, sizes, and timings to create a dynamic and engaging display. Firework designers carefully choreograph the sequence of explosions, incorporating a variety of firework types and effects to tell a story or evoke a particular emotion.
 Firework Display Design and Choreography
Firework Display Design and Choreography
How to do 3 alternating color nails american flag theme? This showcases the creativity in combining colors, similar to designing a firework display. Both require careful planning and execution to achieve the desired visual impact.
Conclusion: The Colorful Chemistry of Fireworks
From the vibrant reds of strontium to the brilliant greens of barium, the colors of fireworks are a testament to the power of chemistry and the artistry of firework designers. By carefully selecting and combining metal salts, these skilled professionals transform the night sky into a canvas of breathtaking color and light, creating memories that last a lifetime. So, the next time you witness a firework display, remember the intricate science and artistry behind each burst of color.
FAQ
- What chemicals make fireworks explode? Fireworks explode due to a reaction between a fuel, such as black powder, and an oxidizing agent.
- What metal makes fireworks blue? Copper salts are responsible for the blue color in fireworks.
- Are fireworks dangerous? Fireworks can be dangerous if not handled properly. Always follow safety guidelines and local regulations.
- How are fireworks made? Fireworks are made by carefully combining various chemicals, including metal salts, fuels, binders, and oxidizing agents, within a shell.
- What is the purpose of fireworks? Fireworks are primarily used for celebrations and entertainment.
- How high do fireworks go? The height of a firework depends on its size and type, but they can reach hundreds of feet into the air.
- What are the different types of fireworks? There are many types of fireworks, including aerial shells, rockets, fountains, and sparklers.
Need assistance with your next project? Contact us at Phone: 0373298888, Email: [email protected] or visit us at 86 Cau Giay, Hanoi. We have a 24/7 customer service team ready to help.

